News
Personalized Gene Delivery to the Gut
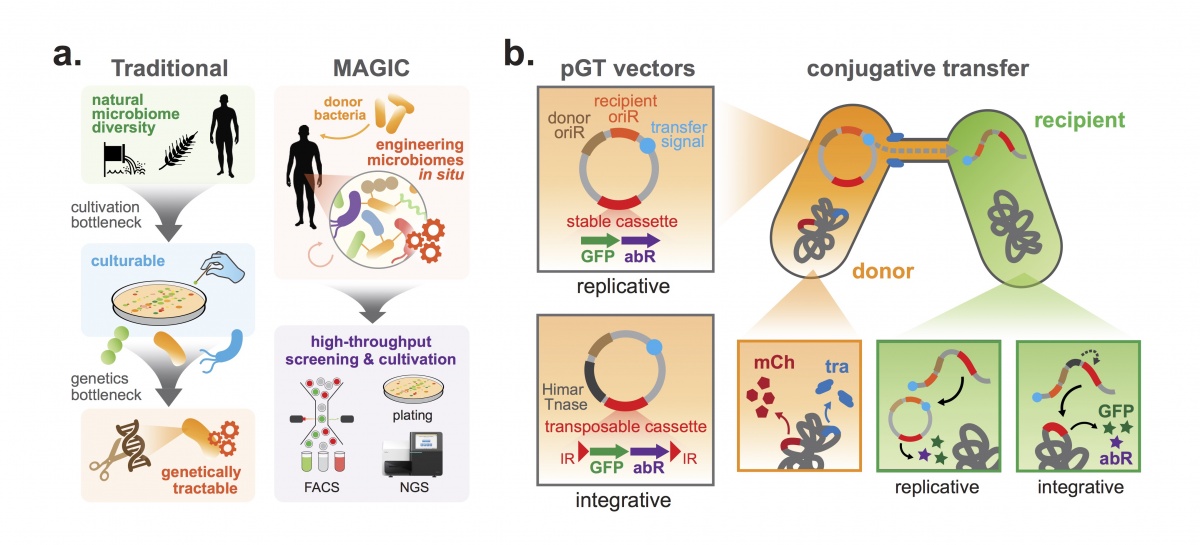
Illustrated here: (a) In contrast to traditional approaches to cultivate microbes first and then test for genetic accessibility, MAGIC harnesses horizontal gene transfer in the native environment to genetically modify bacteria in situ. (b) MAGIC implementation to transfer replicative or integrative pGT vectors from an engineered donor strain into amenable recipients in a complex microbiome. Replicative vectors feature a broad-host range origin of replication (oriR), while integrative vectors contain a transposable Himar cassette and transposase. The donor E. coli strain contains genomically integrated conjugative transfer genes (tra) and a mCherry gene. Transconjugant bacteria are detectable based on expression of an engineered payload that includes GFP and an antibiotic resistance gene (abr).
A team of researchers, led by Dr. Harris Wang of the Department of Systems Biology , has engineered bacteria to benefit and improve the overall health of our gut microbiome. In a proof-of-concept paper published in Nature Methods , Dr. Wang and his team demonstrate MAGIC, an innovative gene delivery system that ‘hacks’ the gut microbiome to perform any desired function, from harvesting energy from food and protecting against pathogen invasion to bolstering anti-inflammatory properties and regulating immune responses.
“The MAGIC system allows us to insert new gene functions directly into an existing microbiome without permanently altering the composition of the microbiome as a whole,” says Sway Chen , an MD/PhD student in the Wang lab and co-author of the study.
“With this system, we can instruct your own bacterial community to express the genes we’ve pre-programmed that are most beneficial to you,” says Carlotta Ronda , PhD, a Simons Junior Fellow, who is the co-lead author working with Chen on the research in the Wang lab . “Your own community will be transformed and make you healthier.”
In recent years, scientists have made major headway in better understanding the gut’s bacterial community, and some of the known benefits of bacteria have made its way to the mainstream. Probiotics, for one, have earned its place in the average household medicine cabinet for those who want to take “good” bacteria to “settle” their stomachs. However, the gut microbiome encompasses far more than our digestive tract.
“The gut microbiome is an intrinsic part of our physiology, and has become increasingly relevant in just the past decade as we continue to uncover the various roles that it plays in overall human health,” notes Dr. Wang, assistant professor of systems biology at Columbia’s Vagelos College of Physicians and Surgeons. “If we want to better understand human physiology, we need to investigate the intricacies of microbiome-host interactions.”
"We can instruct your own bacterial community to express the genes we’ve pre-programmed that are most beneficial to you." -Carlotta Ronda, PhD, study co-author.
For instance, the gut microbiome has been linked to brain chemistry and to the development of our immune system. Studying the complex, multifaceted community of bacteria means not only connecting the dots on how the gut microbiome helps us to digest food, but its involvement in multiple biological mechanisms that have yet to be uncovered.
MAGIC is built on the mechanism of DNA exchange that is naturally present in bacteria. The system is based on conjugation, the process in which bacteria come together naturally to exchange genetic material, a union of sorts. The Wang lab engineered MAGIC as a gene delivery system that repurposes conjugation in the gut microbiome in order to deliver a pre-defined genetic payload (function). Researchers tested in mice model the delivery of a library of vectors carrying a new synthetic function. The actual new genetic trait is directly programmed into the host’s own microbiota through horizontal gene transfer.
In the proof-of-concept study, researchers used an engineered E.coli strains (donor strain) to deliver, via mobile elements, a fluorescent reporter that helped them to identify the genetically modified commensals in the gut. This tracking system enables the isolation and cultivation of gut bacteria carrying the new synthetic function that can then be deployed back as “personalized probiotic”. These host-adapted bacteria were able to infiltrate and stably colonize the existing microbiome acting as a genetic reservoir of the new trait as well as a new donor able to mobilize the engineered DNA. This host personalized strategy allowed the new programmed function to persist for a longer time.
“We exploit this mechanism by re-coding part of the DNA that is being exchanged,” explains Dr. Ronda. “If you can imagine, in the future, we could use this system to introduce anti-cancer drugs that could be microbially encoded, or deliver pathways that produce relevant molecules for human health.”
To this end, the team has been working on re-coding a pathway that promotes gut health and could prevent or cure diseases. Having the ability to deliver a specific pathway for anti-inflammatory effects, for example, into an individual’s gut microbiome could help naturally boost the beneficial properties to the irritated digestive tract. The team is testing this technique in the lab now.
Ongoing work in the Wang lab has included the development of an innovative suite of experimental tools and techniques to understand the gut microbial community at high resolution. Their ongoing work in exploring the mechanisms underlying host-bacteria interactions will provide a deeper understanding of the role the microbiome plays in health and disease.
Says Chen, “We’ve been working on synergistic projects such as understanding how gene regulatory elements function across different bacteria, CRISPR-based memory switches, and encoding two genes in the same piece of DNA, that may come in handy down the road for developing sophisticated genetic circuits in various gut microbes.”
While their current aim with the MAGIC gene delivery system is broad, the researchers foresee this technique to help advance the goal of precision medicine, tailoring their system to target very specific functions beneficial to the gut.
The study, “Metagenomic Engineering of the Mammalian Gut Microbiome In Situ”, was published in Nature Methods. Coauthors include Vitor Cabral of Columbia University and Stephanie J. Yaung of MIT. The work was funded by DARPA, NIH, NSF, ONR, and Burroughs Wellcome PATH.
-Melanie A. Farmer